Optimization of the structural characteristics of CaO and its effective stabilization yield high-capacity CO2 sorbents
In the work reported here, we have developed CaO microspheres featuring structural characteristics which affect very favourably their CO2 uptake.
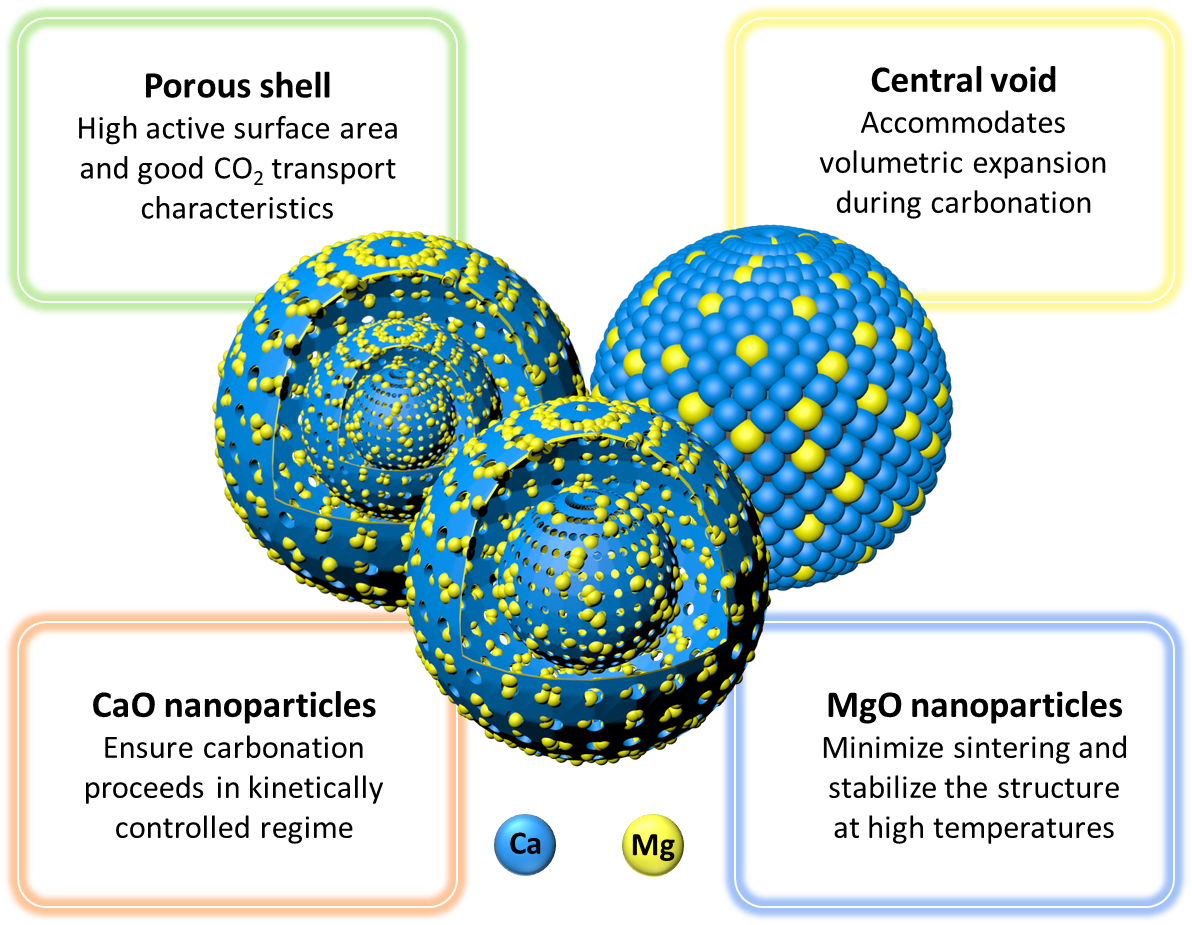
These CaO microspheres exhibit a hollow spherical morphology, whereby the central void is able to accompany the large volumetric expansion of the material during CO2 capture. The central void is realized through the removal of a sacrificial carbonaceous template derived from in situ hydrothermal treatment of xylose. The shell of the hollow spheres is composed of an assembly of CaO nanoparticles (i.e., < 100 nm) which effectively minimize the diffusive transport limitations of CO2 through the growing product layer of CaCO3 during the carbonation process and thus, maintain the inherently fast carbonation kinetics.
We have selected MgO as a stabilizer owing to its high thermal stability and more importantly, due to the fact that it does not form a solid solution with CaO under operating conditions, as opposed to Al2O3, a commonly used stabilizer, which reacts with CaO during cyclic operations and forms a solid solution that is inactive for CO2 capture. Incorporation of MgO into the structure was carried out in situ during hydrothermal synthesis, enabling a single-step synthesis protocol which opens up the opportunity to produce larger quantities of material at a substantially reduced cost. The synthesized sorbent with a MgO content as low as 11 wt. % demonstrated a CO2 uptake of 0.50 gCO2/gCaO after 30 carbonation and regeneration cycles, corresponding to a capacity retention of 83% and surpassing the CO2 uptake capacity of the limestone benchmark by more than 500%.